Priorities for Plane Drawings of Cyclcohexane
6.3: Absolute Configuration and the (R) and (S) System
- Page ID
- 45152
Learning Objective
- proper noun chiral compounds using (R) & (S) nomenclature
USE YOUR MODELING KIT: Models assistance in visualizing the structure. When using a model, make sure the lowest priority is pointing abroad from y'all. Then decide the direction from the highest priority substituent to the lowest: clockwise (R) or counterclockwise (Due south).
IF You lot Exercise NOT Take A MODELING KIT: recall that the dashes mean the bond is going into the screen and the wedges means that bond is coming out of the screen. If the lowest priority bond is non pointing to the dorsum, mentally rotate it and then that it is. However, it is very useful when learning organic chemical science to use models.
If yous have a modeling kit use it every bit you read through this section and work the do problems.
Introduction and the Cahn-Ingold-Prelog rules of Priority
To name the enantiomers of a chemical compound unambiguously, their names must include the "handedness" of the molecule. The letters "R" and "South" are determined past applying the Cahn-Ingold-Prelog (CIP) rules. The optical activeness (+/-) can also exist communicated in the name, but must be empirically derived. There are also biochemical conventions for carbohydrates (sugars) and amino acids (the edifice blocks of proteins).
The method of unambiguously assigning the handedness of molecules was originated by three chemists: R.Due south. Cahn, C. Ingold, and V. Prelog and, as such, is besides frequently called the Cahn-Ingold-Prelog rules. In addition to the CIP system, in that location are two ways of experimentally determining the absolute configuration of an enantiomer:
- X-ray diffraction analysis. Note that there is no correlation between the sign of rotation and the structure of a particular enantiomer.
- Chemical correlation with a molecule whose structure has already been adamant via 10-ray diffraction.
Still, for not-laboratory purposes, it is beneficial to focus on the R/S system. The sign of optical rotation, although different for the ii enantiomers of a chiral molecule,at the same temperature, cannot be used to constitute the accented configuration of an enantiomer; this is because the sign of optical rotation for a particular enantiomer may change when the temperature changes.
The Cahn-Ingold-Prelog rules of priority are based on the atomic numbers of the atoms of interest. For chirality, the atoms of involvement are the atoms bonded to the chiral carbon.
- The atom with higher diminutive number has college priority (I > Br > Cl > South > P > F > O > N > C > H).
- When comparing isotopes, the atom with the college mass number has higher priority [18O > xviO or fifteenDue north > 14Due north or 13C > 12C or T (3H) > D (2H) > H].
- When in that location is a necktie in (2) in a higher place, establish relative priority by proceeding to the next cantlet(south) along the chain until the first difference is observed.
Multiple bonds are treated as if each bond of the multiple bond is bonded to a unique cantlet. For case, the ethenyl group (CH2=CH) has higher priority than the ethyl group (CH3CH2). The ethenyl carbon priority is "two" bonds to carbon atoms and one bond to a hydrogen cantlet compared with the ethyl carbon that has simply one bail to a carbon atom and two bonds to two hydrogen atoms. Similarly, the carbon-carbon triple bond of acetylene would give it higher CIP priority than the ethenyl group as summarized below.
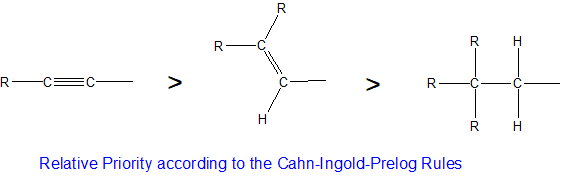
Stereocenters are labeled R or Southward
The "right paw" and "left paw" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labeled as R or S.
Consider the diagram above on the left: a curved arrow is drawn counter-clockwise (c-cw) from the highest priority substituent (one) to the lowest priority substituent (four) in the Due south - configuration ("Sinister" → Latin= "left"). The counterclockwise direction can be recognized past the motility left when leaving the 12 o' clock position. At present consider the diagram higher up on the right where a curved pointer is drawn clockwise (cw) from the highest priority substituent (ane) to the everyman priority substituent (4) in the R configuration ("Rectus" → Latin= "correct"). The R or Southward is then added as a prefix, in parenthesis, to the name of the enantiomer of involvement. A locator number is required if in that location is more one chiral centre. Otherwise, the person reading the name is expected to recognize the chiral center.
Example ane
The two chiral compounds below are drawn to emphasize the chiral carbon with the full chemical proper noun below each construction.
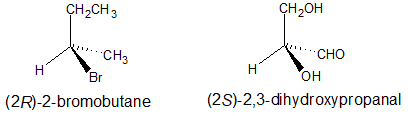
Accented Configurations of Perspective Formulas
Chemists need a convenient way to distinguish 1 stereoisomer from another. The Cahn-Ingold-Prelog system is a fix of rules that allows usa to unambiguously ascertain the stereochemical configuration of whatever stereocenter, using the designations ' R ' (from the Latin rectus, meaning right-handed) or ' S ' (from the Latin sinister, meaning left-handed).
The rules for this system of stereochemical nomenclature are, on the surface, fairly simple.
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/06%3A_Stereochemistry_at_Tetrahedral_Centers/6.03%3A_Absolute_Configuration_and_the_(R)_and_(S)_System
0 Response to "Priorities for Plane Drawings of Cyclcohexane"
Post a Comment